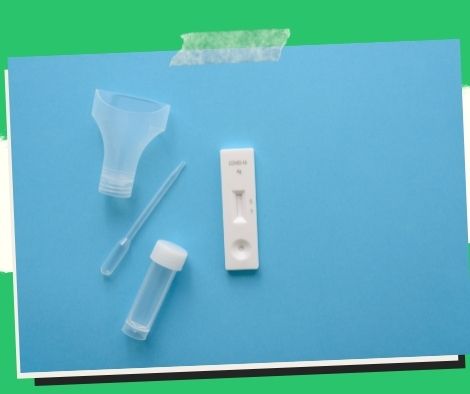
When self-test kits are used incorrectly, the findings can be inaccurate.
If not utilized correctly, self-administered or home test kits for Covid-19 will produce erroneous results.
Health Secretary Francisco Duque III stated that the standards for the use of antigen kits are being consolidated to minimize false negative or positive results.
“‘Yung mga self-administered test kits, may mga kanya-kanyang pamamaraan, ‘yung product label na nakalagay dun kung paano gagamitin, so kinakailangan maging angkop ‘yung guidelines doon sa mga instructions ng mga antigen test kits. The product label will tell you how to use it, so double-check that the parameters match the antigen test kit instructions) “In a Friday interview, Duque stated.
He went on to say that the established standards would also cover how to properly dispose of the test kits.
The Food and Drug Administration (FDA) has previously approved the use of two Covid-19 home self-testing antigen kits, manufactured by Abbott and Labnovation Technologies Inc. and sold in FDA-licensed drug stores.
Nasal, nasopharyngeal, and/or oropharyngeal samples are used in antigen test kits.
Individuals with positive fast antigen test results should be separated and managed as Covid-19 cases until a confirmation test is performed, according to the DOH.
Those with a high index of suspicion and who tested negative on quick antigen tests should be isolated until a reverse transcription-polymerase chain reaction (RT-CR) test or repeat antigen results can establish their negative status.
Within 48 to 72 hours of the initial antigen test, a confirmatory test should be performed.
The antigen test’s positive result can be used to isolate patients with suspected infection early, however it cannot be utilized to diagnose SARS-CoV-2 severe acute respiratory syndrome coronavirus-2 infection.
Negative results do not rule out the possibility of SARS-CoV-2 infection and should not be used to guide treatment.
“For suspected populations whose antigen test results are positive or negative, further nucleic acid testing should be carried out,” Labnovation Technologies wrote on its website.
Save/Share this story with QR CODE
Disclaimer
This article is for informational purposes only and does not constitute endorsement of any specific technologies or methodologies and financial advice or endorsement of any specific products or services.
 Need to get in touch?
Need to get in touch?

We appreciate your reading. 
1.) 

Your DONATION will be used to fund and maintain NEXTGENDAY.com
Subscribers in the Philippines can make donations to mobile number 0917 906 3081, thru GCash.
3.) 
4.) 
AFFILIATE PARTNERS

World Class Nutritional Supplements - Buy Highest Quality Products, Purest Most Healthy Ingredients, Direct to your Door! Up to 90% OFF.
Join LiveGood Today - A company created to satisfy the world's most demanding leaders and entrepreneurs, with the best compensation plan today.

 Business, Finance & Technology
Business, Finance & Technology





